Iodine is a chemical element with the symbol I and atomic number 53.The element was discovered by the French chemist Bernard Courtois in 1811. It was named two years later by Joseph Louis Gay-Lussac from this property, after the Greek ἰώδης "violet-coloured".
Today, iodine is chiefly obtained from deposits of sodium iodate (NaIO3) and sodium periodate (NaIO4) in Chile and Bolivia. Trace amounts of iodine are required by the human body. Iodine is part of thyroxines, a hormone produced by the thyroid gland that controls the body's rate of physical and mental development.
Iodine is fairly rare in both the Earth's crust and in the solar system. Iodine is the 47th most abundant element in the solar system. It is the 60th most common element in the Earth's crust .
Iodine is taken by mouth to prevent and treat iodine deficiency and its consequences, including goiter and some thyroid disorders. It is also used for treating lumpy breasts (fibrocystic breast disease) and breast pain (mastalgia).
Iodine is likely safe for most people when taken by mouth in recommended amounts or when applied to the skin appropriately using approved products. Iodine can cause significant side effects in some people. Common side effects include nausea and stomach pain, runny nose, headache, metallic taste, and diarrhea.
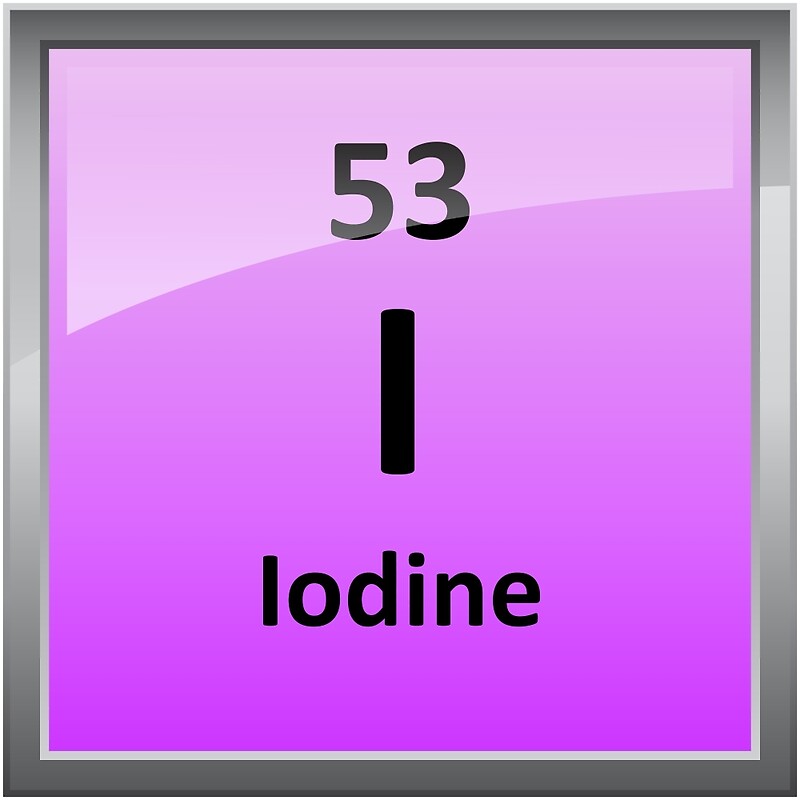
Atomic number:53
Symbol: I
Origin of name: Iodes
Electrons per shell : 2, 8, 18, 18, 7
Discoverer: Barnard Courtois
Isolator: Barnard Courtois
Iodine - Atomic mass: 124.90447 u
-Density: 4.94 g/mL
-Type: Other nonmetals
Isolation Process : Barnard Courtois was extracting sodium and potassium compounds from seaweed ash. Once these compounds were removed, he added sulfuric acid (H2SO4) to further process the ash.
Half Life : 8.02 days
Melting Point :113.5°C
 |
| Iodine Isotope |
Amount of Isotopes: 37
Isotopes’ Types: 108I , 144I , 127I
Isotopes’ Definition :
108I 127I 144I
Proton : 53 Proton : 53 Proton : 53
Neutron : 55 Neutron : 74 Neutron : 91
Electron : 53 Electron : 53 Electron : 53
Chemical Properties:
> Melts to form a deep violet liquid at 114 degrees Celsius
> Boils to a violet gas at 184 degrees Celsius
> Both radioactive and non-radioactive forms
Physical Properties:
> Purple-black non-metallic solid
> Odor and corrosion poison
> Will sink in water
Uses
> Used in medicines
> Used as a disinfectant for external wounds
No comments:
Post a Comment